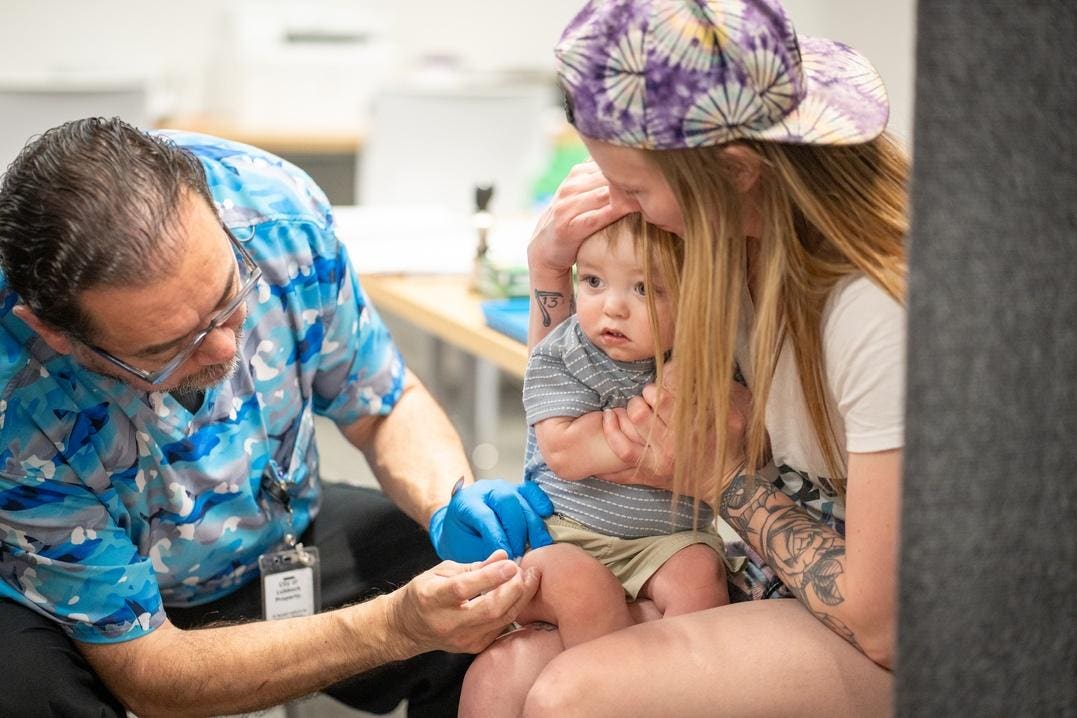
Washington DC – On Monday, Sept. 22, 2025, President Donald Trump said his administration was linking acetaminophen, the active ingredient in Tylenol, to autism and urging pregnant women to largely avoid the medication. At the same time, the Food and Drug Administration is approving use of leucovorin for autism. Photographer: Francis Chung/Politico/Bloomberg
© 2025 Bloomberg Finance LP
The Trump administration announced late last month “bold actions to tackle the autism epidemic.” President Trump said there was a possible link between acetaminophen, the active ingredient in Tylenol, and autism. Controversially, he urged pregnant women to largely avoid the medication. At the same time, the Food and Drug Administration announced it is approving use of leucovorin (folinic acid) calcium tablets for cerebral folate deficiency, hypothesized to be a contributing factor in some individuals with autism spectrum disorder. The science, however, is far from settled with respect to leucovorin’s ability to treat symptoms in autistic patients who have cerebral folate deficiency.
Leucovorin is a drug that hasn’t been formally marketed in 25 years. Nevertheless, it is commonly prescribed to mitigate the side effects of certain medications used in cancer, such as methotrexate. The FDA is now asking the sponsor GSK to update the label to reflect its authorization as a treatment of cerebral folate deficiency.
But a piece in MedPage Today says that the treatment doesn’t have evidence from large randomized controlled trials supporting its use in patients with autism. Moreover, a connection between cerebral folate deficiency and autism remains unclear.
Cerebral folate deficiency is a rare condition in which a person’s brain isn’t getting enough vitamin B9. The Cleveland Clinic describes folate as a “B vitamin that your body needs to work properly.” It goes on to say that folate is especially important if pregnant.
Symptoms from a folate deficiency include intellectual disability, lack of muscle control and seizures. Some of the symptoms overlap those of autism spectrum disorder. An FDA statement indicates leucovorin’s approval would be for people with cerebral folate deficiency. While this condition may lead to autism-like symptoms and developmental delays, having the deficiency is distinct from autism. Only a minority of people with autism also have cerebral folate deficiency.
FDA Commissioner Marty Makary stated during the White House news conference last week that he thinks “hundreds of thousands of kids with autism” could benefit from taking the drug, specifically improvements in speech-related deficits for a subset of children. Yet, based on the evidence thus far, it’s far from certain that this would happen.
The National Institutes of Health is unveiling new programs that include research into causes and treatments for autism. And in a notice posted to the Federal Register in September, the FDA wrote that it had conducted a systematic analysis of studies published between 2009 and 2024 and found that leucovorin was shown to improve speech-related deficits.
Richard Frye, chief scientific officer of the Autism Discovery and Treatment Foundation, is responsible for several important studies on leucovorin. He has told media outlets that children with autism often have an antibody that blocks folate from being transported into the brain. Frye says that preliminary data suggest leucovorin could help improve communication skills and other symptoms associated with autism. To illustrate, in 2013, Frye published a study involving 48 patients, which demonstrated that children with autism as well as those with cerebral folate deficiency could benefit from the drug. In the study, up to 75% of children with autism showed deficiencies in brain folate. Children treated with leucovorin improved their language, interpersonal, social and coping skills. Five years later, Frye confirmed those findings in another small-scale study that compared children receiving leucovorin to a group getting a placebo drug.
STAT News reports that some doctors already prescribe leucovorin off label to patients with autism. Nevertheless, in a follow-up article on Oct. 1, the publication interviewed a leucovorin researcher who believes benefits are modest and that parents’ expectations shouldn’t be raised too high.
The Autism Science Foundation issued a statement saying a “much higher standard of science would be needed to determine if leucovorin is an effective and safe treatment for autism. This science is still in very early stages, and more studies are necessary before a definitive conclusion can be reached.”
The American Psychiatric Association was more emphatic, stating that leucovorin has not been a recommended therapy for autism and it “will require many more years of research before we know if leucovorin is an appropriate treatment for individuals with autism.”
What makes matters complex with respect to autism is the extraordinary difficulty of teasing out the mix of various genetic and environmental factors that are thought to be causal. In turn, this may make seemingly simple solutions like leucovorin problematic.